 Another paper from Vishwanath’s old work in India was just published in the very good Journal of Materials Chemistry A. Brilliant work!
Another paper from Vishwanath’s old work in India was just published in the very good Journal of Materials Chemistry A. Brilliant work!
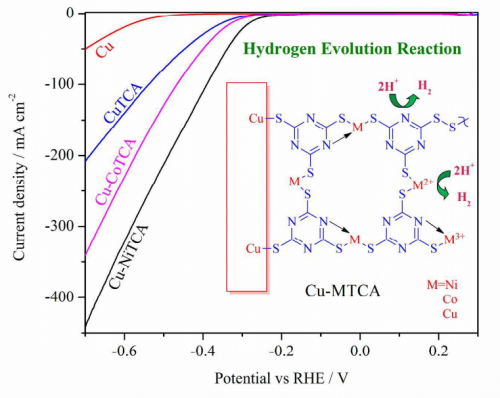
The paper concerns the creation of electrocatalysts for the hydrogen evolution reaction, something that is urgently needed if hydrogen shall be produced using sustainable amounts of energy.
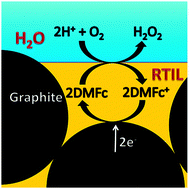
The acid-stable and efficient non-noble metal-organic hybrid electrocatalytic surfaces are on focus for hydrogen evolution at low overpotentials. In this manuscript, we describe the electrochemical preparation of novel chemically immobilized mixed valent /mixed-metal ions containing triazine thiolate polymer electrocatalysts on the copper surface. These metallopolymeric thin films exhibit substantial overpotential reduction, a stable and an improved hydrogen evolution activity in strong acidic electrolytes.We have investigated these electrocatalytic thin films by electrochemical and other surface analytical techniques. The surface distribution of active sites play an important role in the three phase heterogeneous electrocatalysis.