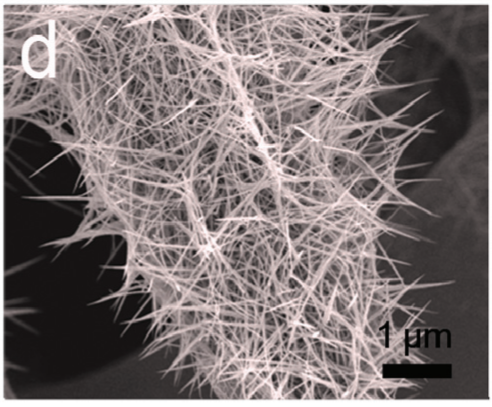
Another article by Vishwanath in his collaboration with his old Indian institute has just been published in Journal of Catalysis. In this paper they have shown a facile method for preparing thin films of Ruthenium ion containing metallopolymers chemically immobilised on the reactive electrode substrate. These electrodes exhibit a remarkable overpotential reduction with an excellent acidic stability as a thin film electrocatalyst in three phase heterogeneous gas evolution reaction.
Another article by Vishwanath in his collaboration with his old Indian institute has just been published in Journal of Catalysis. In this paper they have shown a facile method for preparing thin films of Ruthenium ion containing metallopolymers chemically immobilised on the reactive electrode substrate. These electrodes exhibit a remarkable overpotential reduction with an excellent acidic stability as a thin film electrocatalyst in three phase heterogeneous gas evolution reaction.
In the paper the auhtors propose high catalytic the activity could be achieved by the selective design of these types of nitrogen and sulphur rich triazine units and modulation of coordination environment with the redox active metal centers. The approach of using the functional linker ligands with excellent coordination capability with transition metals, good electronic transport characteristic, and dispersion of active sites could have huge potentials in other electrocatalytic process. Furthermore, these metallopolymers are directly fabricated on selective electrode substrates and this eliminates the need of any additional binders and could reduces the potential drop at the substrate/electrocatalyst interface.