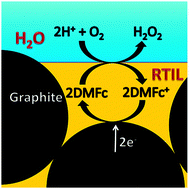
Martin had a part in a new paper that was just published in Electrochimica Acta1. The paper is mainly the work of our previous group member Marcin Filipiak performed in the company BioMedX in Heidelberg, but it builds on some of the work Marcin performed in our group before he moved to Germany.
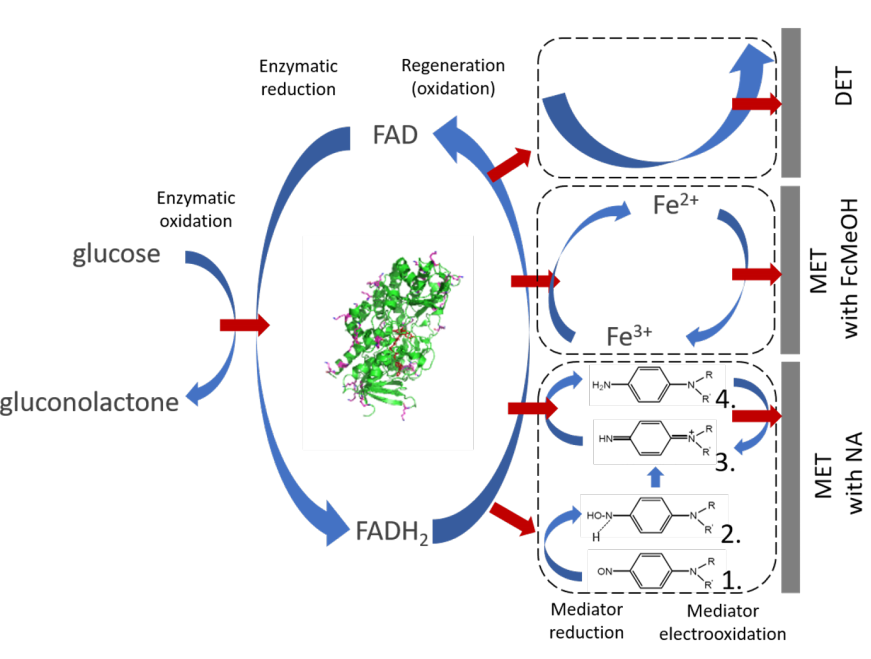
In the paper electrodes of single-sheet graphene were used in a flow-cell to investigate the electron transfer from the enzyme glucose dehydrogenase using two different mediators as well as without mediator. The enzyme in this case was anchored to the graphene surface using pyrenes with a short linker chain. The linker was covalently bonded to the enzyme while the pyrene attached to the surface through π-electron interaction. We used both the standard mediator ferrocenemethanol (FcMeOH) and a novel nitrosoaniline derivative that is also used in glucose sensors produced by Roche.
The FcMeOH, which is a simple redox system, gives the highest signal from the oxidation of glucose, but as expected we see a very strong interference from ascorbic acid. Nitrosaniline on the other hand has a more complex reaction, mechanism that is discussed in the paper, is more robust. In the presence of ascorbic acid the signal has a constant offset, but the response to glucose oxidation on top of this offset is very similar to the case without the acid present.
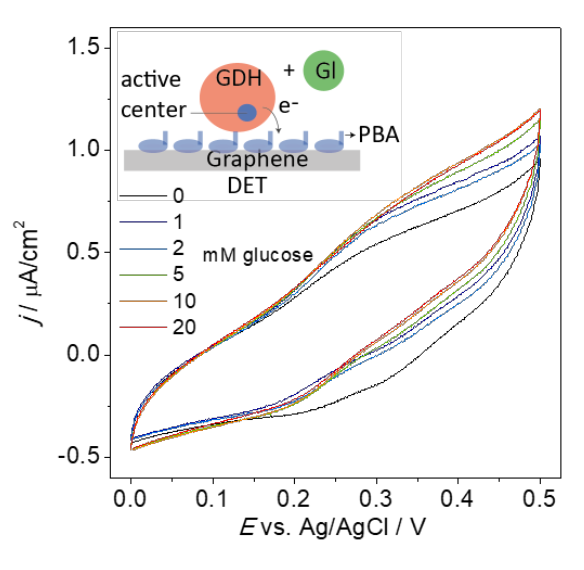
A very important part of the paper was the observation of non-mediated electron transfer from the enzyme to the electrode. Such non-mediated transfer has previously been observed in GDHs with heme-groups that can function as “internal” mediators. In this case the enzyme contained no heme-groups. We speculate that the short linker holds the enzyme close enough to the surface to DET to take place. Although the oxidation current is quite much smaller than the mediated current this is an interesting result that requires further investigation.
Martin’s involvment in this work was as electrochemistry adviser to the sensor group at BioMedX where Marcin worked. We also wants to take the chance to thank our formed student who successfully defended his PhD theses at the University of Heidelberg shortly before this paper was accepted.
- M. S. Filipiak, D. Vetter, K. Thodkar, O. Gutíerrez-Sanz, M. Jönsson-Niedzółka, A. Tarasov
Electron transfer from FAD-dependent glucose dehydrogenase to single-sheet graphene electrodes, Electrochim. Acta (accepted)(link).