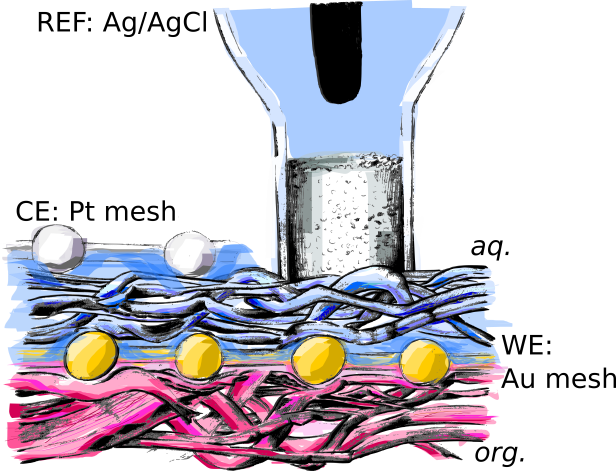
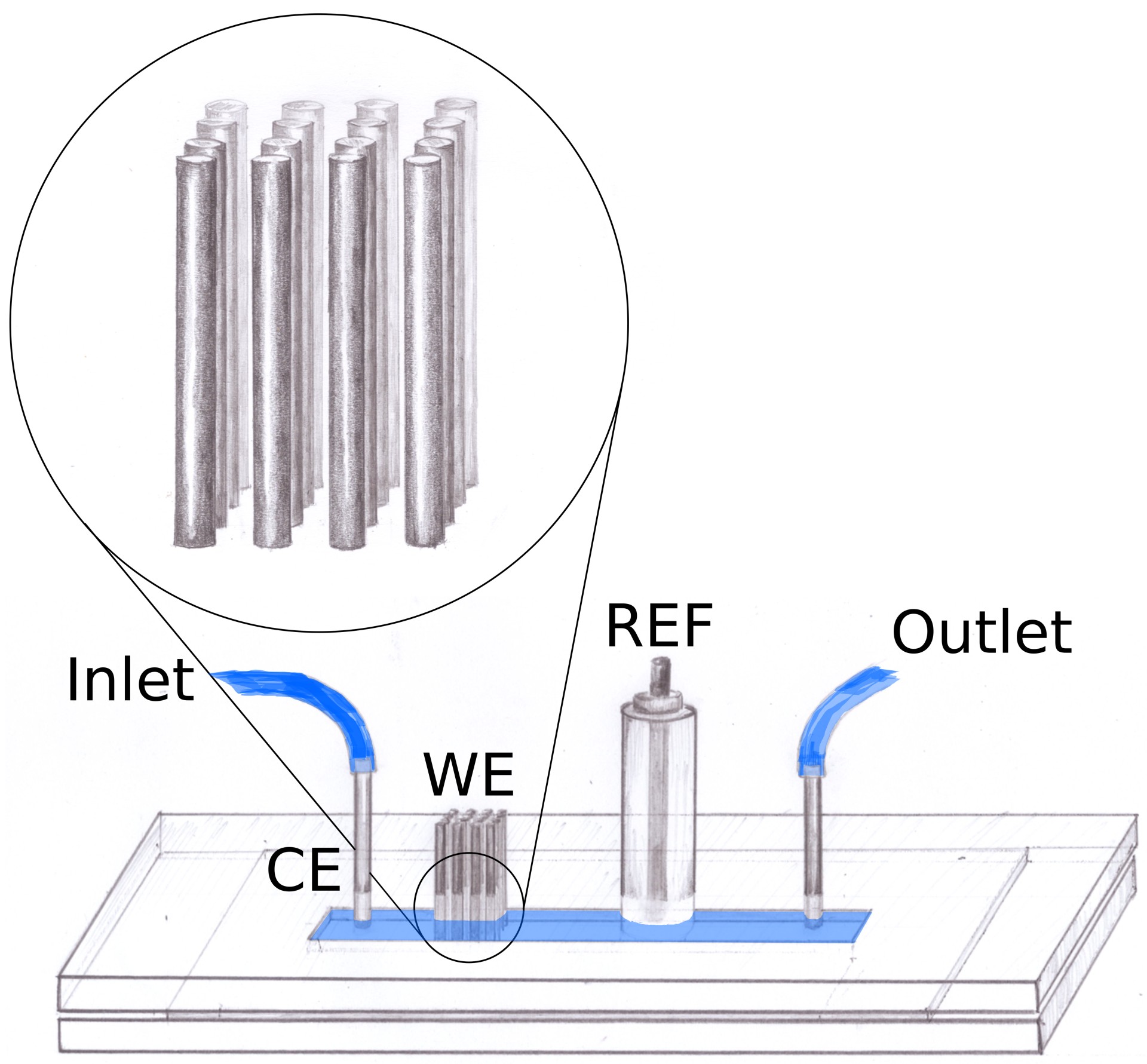
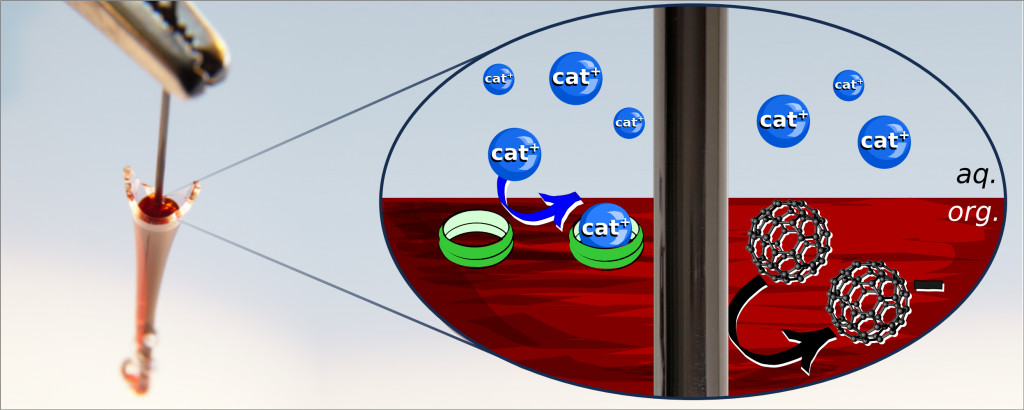
a pencil graphite electrode filled with the organic phase.
We’re on a roll. Another cation transfer paper was accepted1, also in Electrochimica Acta. This is work done by Marta and our undergrad student Julia. They were investigating transfer of cations at the three-phase junction driven by reduction of the fullerene C60 and facilitated by the addition of ionophores to the organic phase.
Normally these kind of measurements are performed in a setup with a droplet on a glassy carbon electrode, but here we created a new setup with a pencil graphite electrodes in a small glass vial. The vial was made by breaking and sealing off the end of a Pasteur pipette. This gave us a more stable three-phase junction to work with.
Adding an ionophore to the organic solution helps “carrying” ions from the aqueous phase to the organic. Since the reduction of the fullerene must be accompanied by ion transfer, to compensate for the charge of the electron injected into the organic phase from the electrode, this will help lower the potential needed to reduce the fullerene. Since ionophores are designed to specifically carry one particular ion, that ion will be transferred easier than others, and from the change in transfer potential with and without ionophore, one can calculate how selective the ionophore is. This is very useful information for ion-selective electrodes. We tested this with three different K+-selective ionophores and used cation transfer at the TPJ to calculate their selectivity and compare it to values from the literature.
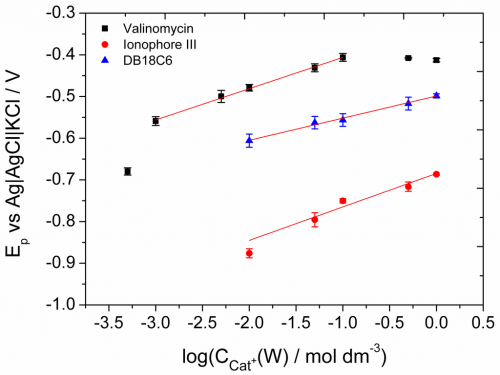
K+ in the presence of 1 mM Valinomycin (black squares), Ionophore III (blue triangles) or DB18C6 (red circles).
One part of the results that we couldn’t quite explain was the dependence of the peak potential on the concentration of the cation in the aqueous phase. This should follow the Nernst-equation, so at room temperature the peak should shift 59 mV if the concentration changes ten times. We measured a higher value that that, and cannot explain why. A lower value would be easier, lots of different losses can cause this… but higher. If anyone has a good idea, we’re all ears.

1 M. Podrażka, J. Maciejewska, W. Adamiak, E. Witkowska Nery, M. Jönsson-Niedziółka
Facilitated cation transfer at a three-phase junction and its applicability for ionophore evaluation, Electrochim. Acta (Accepted). (link – free preprint)