Over the last year, Magda has been working with bachelor student Natalia to investigate the redox behaviour of different β-amyloids.
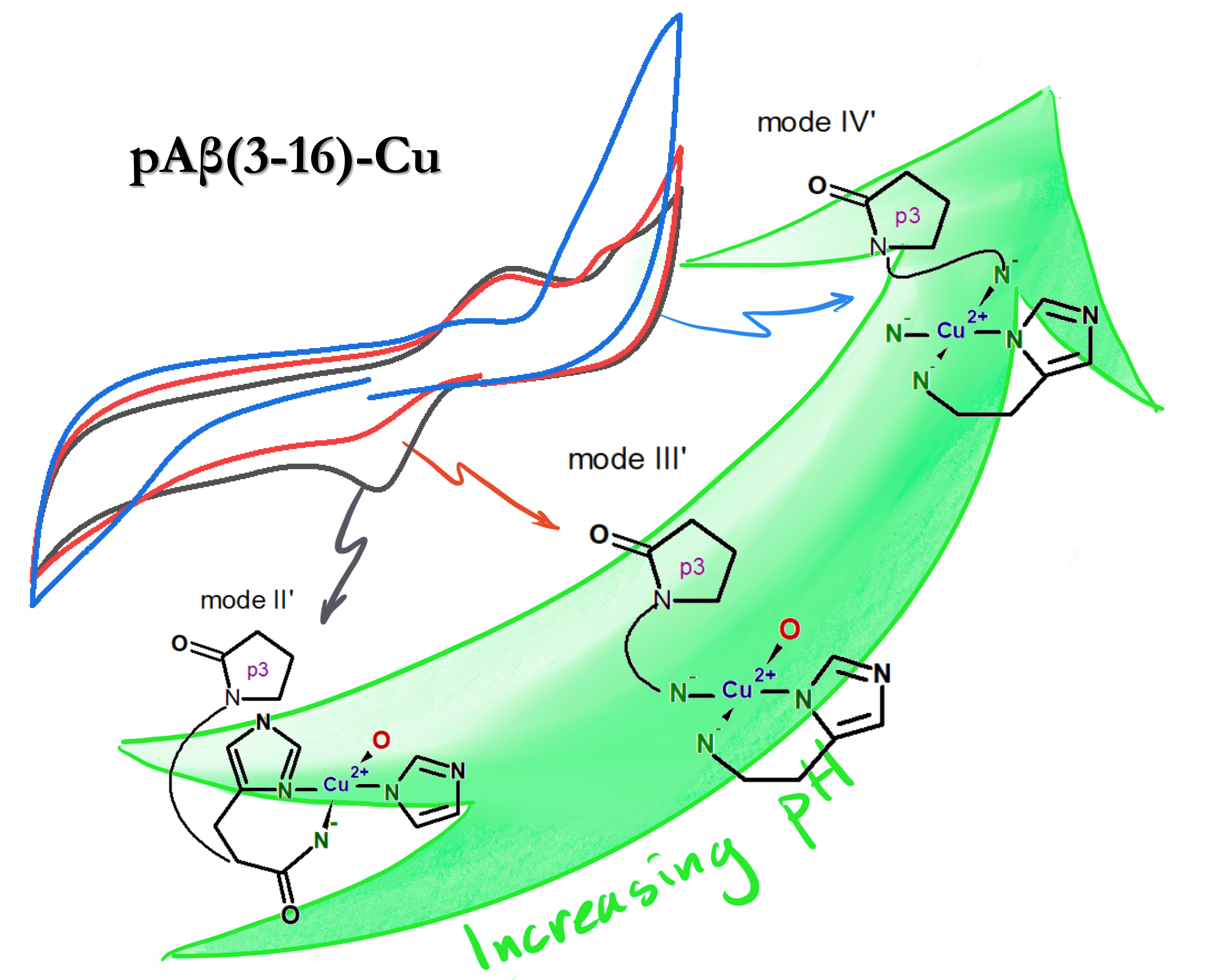
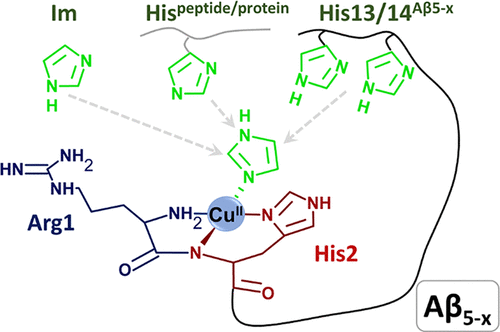
In her recently published paper1 she demonstrates a significant difference in the redox behaviour of copper complexes with peptides associated with Alzheimer’s disease (AD).
AD is a neurodegenerative disorder and senile plaques, comprising different Aβs, are the pathological hallmark of AD. Until now, the most studied amyloid has been Aβ(1–42), which neurotoxicity is related to the formation of reactive oxygen species due to the Cu(II)/Cu(I) reduction process.
In contrast to Aβ(1–42) little is known about Cu(II) complexes with N-terminally modified Aβ which occur in significant proportion in senile plaques. However, in vitro studies show that N-truncated Aβ(3-42) and its pyroglutamate counterpart pAβ(3-42) display faster aggregation compared to Aβ(1–42), and this process is greatly enhanced in the presence of Cu(II) ions. Additionally, it was reported that pAβ(3-42), due to the N-terminal cyclization of the peptide, is not susceptible to the degradation process, which makes it even more toxic.
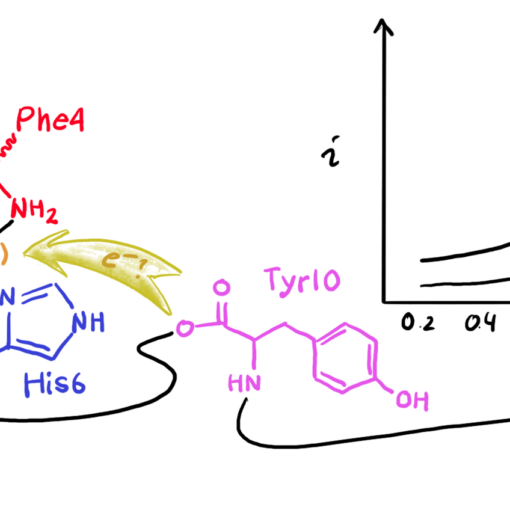
Electrochemical properties of pAβ(3-42) species and its Aβ(3-42) precursor have not been investigated before. In this manuscript, Magda and her co-authors have focused on two important aspects. Firstly, they investigated the redox chemistry of pAβ(3-16)-Cu(II) and Aβ(3-16)-Cu(II) complexes as a model system for pAβ(3-42) and Aβ(3-42) at physiological pH (7.4). Here they showed that both complexes lead to Cu(II)/Cu(I) reaction at potentials associated with increased ROS production. Secondly, they performed experiments in a very wide pH range that draws attention to that changes of the coordination structures significantly influences on the current response.
We believe that these results can be crucial not only for an increased understanding of AD pathogenesis but also contribute to the general understanding of redox reactions that can take place in the human organism as a result of the formation of Cu-peptides.
- M. Z. Wiloch, N. Baran, M. Jönsson-Niedziółka
The Influence of Coordination Mode on the Redox Properties of Copper Complexes with Aβ(3-16) and its Pyroglutamate Counterpart pAβ(3-16), ChemElectroChem (accepted) (link)(ChemRxiv – preprint)

One thought on “The Influence of Coordination Mode on the Redox Properties of Copper Complexes with Aβ(3-16) and its Pyroglutamate Counterpart pAβ(3-16)”