Sometimes publishing doesn’t go according to plan…. Our newly published article in J. Electroanal. Chem.1 was supposed to have been published before the follow-up in ChemElectroChem. We thought it made sense to first study the shorter amyloid and then follow with a study of the longer one. Some review delays and additional experiments made it come out second. We fought long and bravely for it, and finally it’s here!
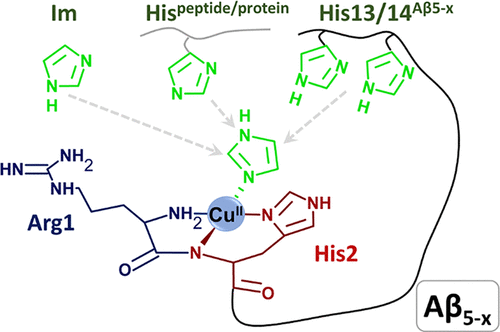
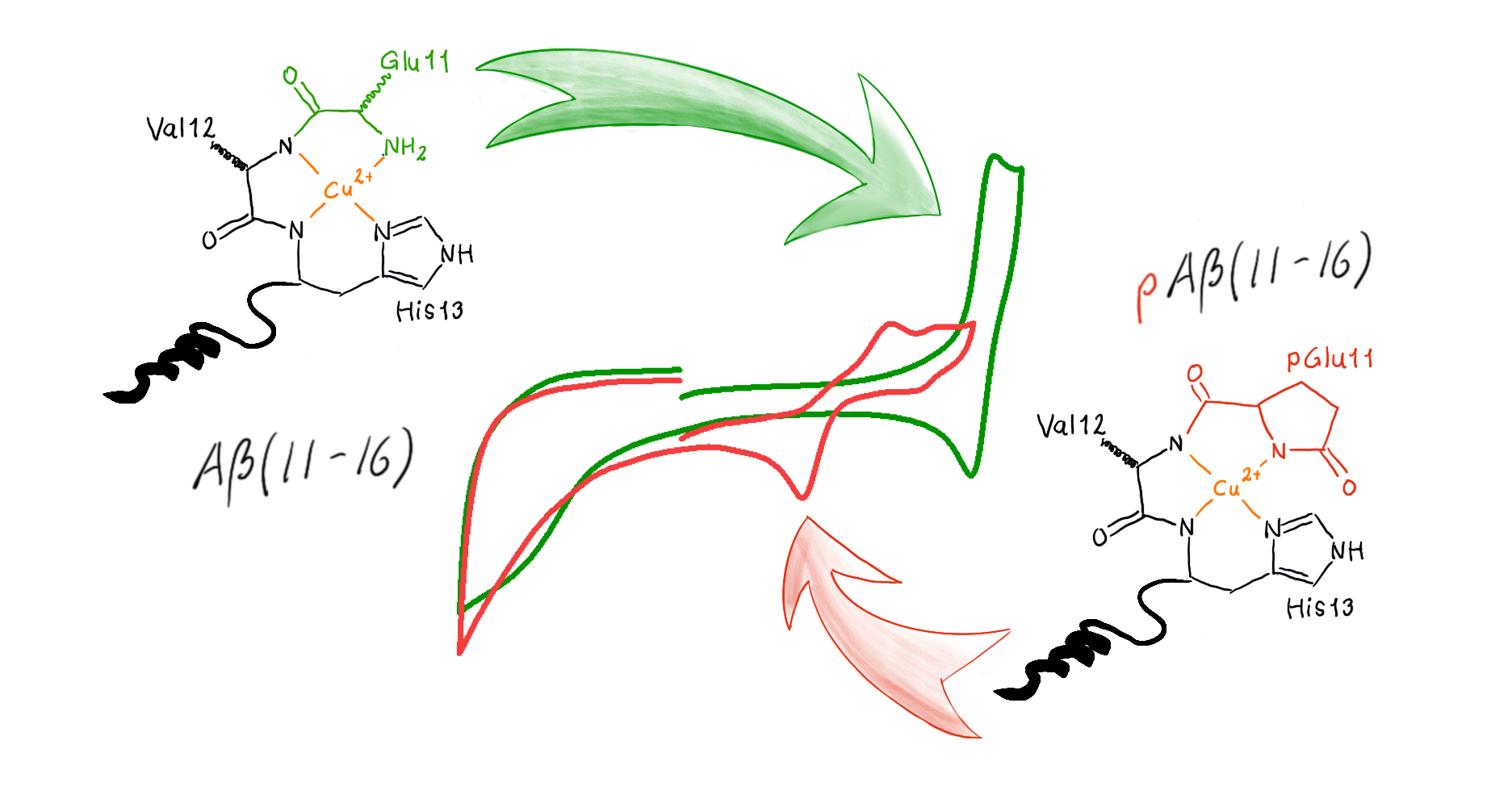
This time, Magda, who studies β-amyloids-Cu complexes, checked how a small change in the structure of another peptide changes the redox properties. This time the choice was a β-amyloid truncated further up the chain, as compared to the previous publication. In Aβ(11-16)-Cu(II) the copper ion is complexed in a very stable 4N binding mode called ATCUN (amino-terminal copper and nickel motif), where it binds to four nitrogen atoms in the peptide chain. This stabilises the Cu(II) ion and suppresses the reduction to Cu(I). However, the transformation of the initial glutamate into a pyroglutamate in pAβ(11-16) removes the ATCUN binding and has lower affinity to the Cu(II) ion. This means that the reduction of Cu(II) to Cu(I) can happen at a potential relevant to conditions in the body. In previous studies, this has been associated with the formation of toxic reactive oxygen and nitrogen species (ROS and RNS). This is also important because the pyroglutamte residue stabilises the peptides against further degradation by enzymes, meaning that the p-versions of the β-amyloids remain longer in the brain and are prone to formation of plaques.
Furthermore, we showed that using a phosphate buffer (that somewhat more resembles the conditions in the body) instead of the commonly used potassium nitrate, further decreases the affinity to Cu(II). This can be caused by interaction between the peptide and the phosphate ion.
The results are interesting not only because of the studied electrochemical properties, but also because they will change the view of the role of β-amyloids in Alzheimer’s disease. Our study also underlines the importance of interactions with other species available in the human body, and the proper choice of electrolyte solutions.
- M. Wiloch, M. Jönsson-Niedziółka
Very small changes in the peptide sequence alter the redox properties of Aβ(11-16)-Cu(II) and pAβ(11-16)-Cu(II) β-amyloid complexes, J Electroanal Chem. (Accepted). (link – OA)(ChemRxiv – preprint)