The next paper1 in Magda’s series of articles on the redox behaviour of β-amyloids relevant for Alzheimer’s disease has just hit the press.
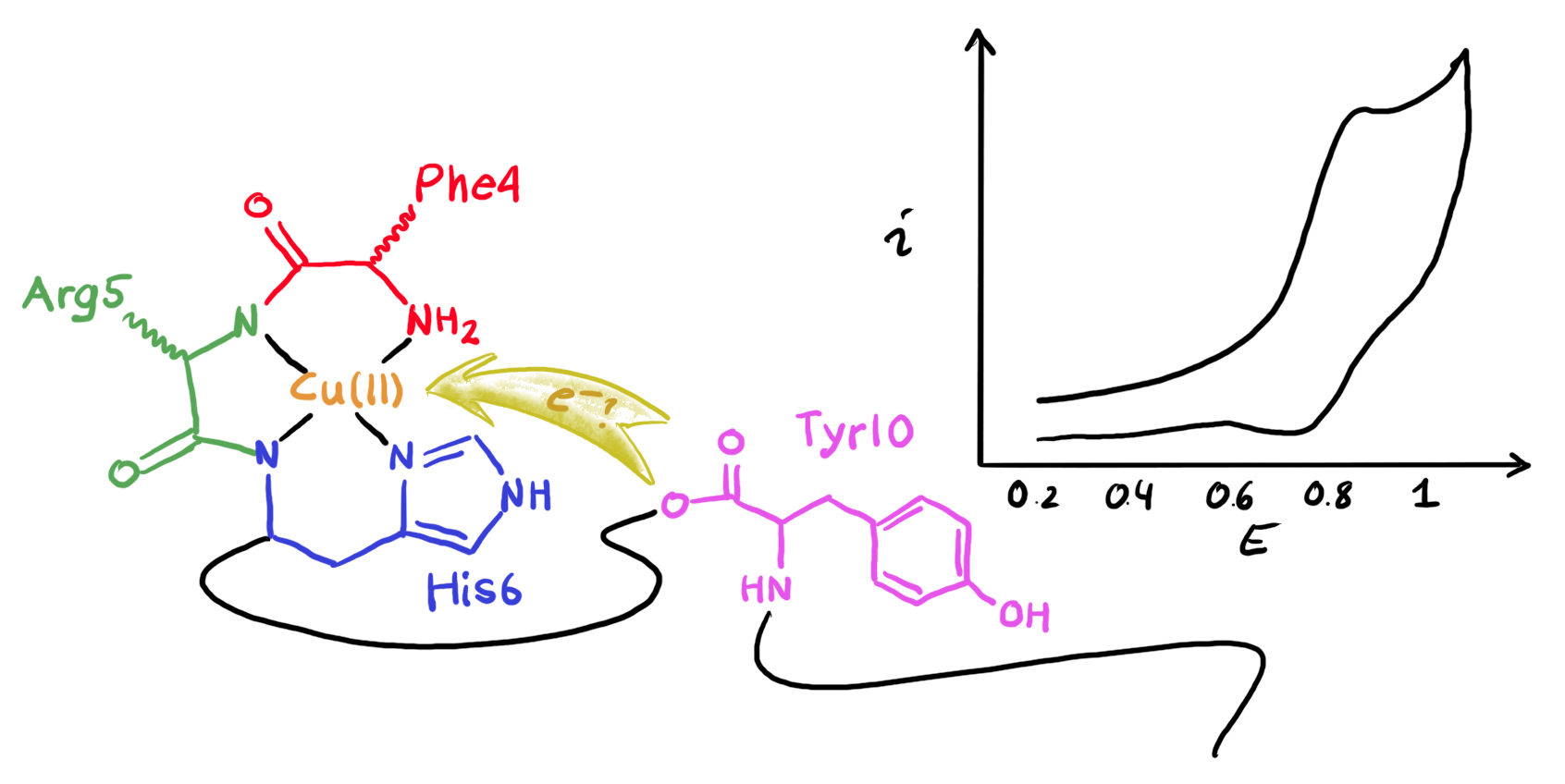
Here we have been looking at how the presence of a redox-active amino acid, tyrosine (Tyr), affects the oxidation of a Cu(II) attached to the amino terminus of Aβ(4-16).
Although Cu(II) ions, bonded by Aβ(4-42), can be oxidized to highly reactive Cu(III) ions, these complexes do not appear to contribute to the formation of reactive oxygen or nitrogen species (ROS/RNS). So, in this paper we look at the pH-dependent voltammetric response of Aβ(4-16)-Cu(II) complexes to understand the influence of the deprotonation of tyrosine within the complex on the Cu(II)/Cu(III) reaction. The results will help us better understand the scavenging role of tyrosine in quenching highly reactive Cu(III) ions not only in Aβ(4-x)-Cu(II) complexes but also provide clues to the reactive properties of other tyrosine-containing amyloid-metal complexes.
Here we worked together with Steven and Wojtek from our neighbouring Nanoelectrochemistry group to do measuements at high scan rate using microelectrodes. We also used square-wave voltammetry, and especially the frequency (scanr rate) dependence of the reverse current to estimate the rate of the internal electrontransfer between Tyr and Cu(III).
- M. Z. Wiloch, S. Linfield, N. Baran, W. Nogala and M. Jönsson-Niedziółka
Redox behaviour of Cu-Aβ(4-16) complexes related to Alzheimer’s Disease, Electrochim. Acta (accepted), (link)(preprint on ChemRxiv).